Mounjaro for Weight Loss and Type 2 Diabetes - a Complete Guide for Patients
Skip to:
Mounjaro is a drug for weight loss and diabetes treatment that contains the substance tirzepatide.
Tirzepatide is the first dual incretin mimetic officially approved by the FDA. It simulatenously mimics two hormones that are involved in appetite control and blood sugar regulation.
Due to its high efficacy and good safety profile, Mundjaro was first approved in the US and then in Europe for the treatment of:
- Obesity (BMI>30kg/m2)
- Overweight (BMI>27kg/m2) accompanied by weight-related metabolic diseases
- Obstructive sleep apnea
- Second-line therapy in people with type 2 diabetes (T2D).
It is important to note that Mounjaro is available only via prescription and is only suitable for people who are overweight and have weight-related conditions such as T2D, high blood pressure, or obstructive sleep apnea.
If you are interested in whether this medication is right for you, be sure to consult a qualified physician with experience in this field before starting any therapy.
What is Mounjaro?
Mounjaro is an officially approved medication containing tirzepatide. Tirzepatide is a peptide developed by the American pharmaceutical company Eli Lilly & Co.
Tirzepatide (the active ingredient in Mounjaro) is a peptide containing 39 amino acids arranged in a sequence that simultaneously mimics parts of the amino acid sequence of two natural human hormones.
These human hormones are the so-called "glucose-dependent insulinotropic peptide" (GIP) and "glucagon-like peptide-1" (GLP-1).
GLP-1 and GIP are naturally secreted by endocrine cells in the intestine, with food intake being the main stimulus for their secretion.
These hormones play a key role in stimulating insulin production and regulating appetite in the human body, but their effect is quite short-lived - only a few minutes [1],[2].
Mounjaro (tirzepatide) works by mimicking these hormones, but the effect persists for extended periods, even after a single dose. Thus, it activates their receptors in your tissues more strongly and sustainably.
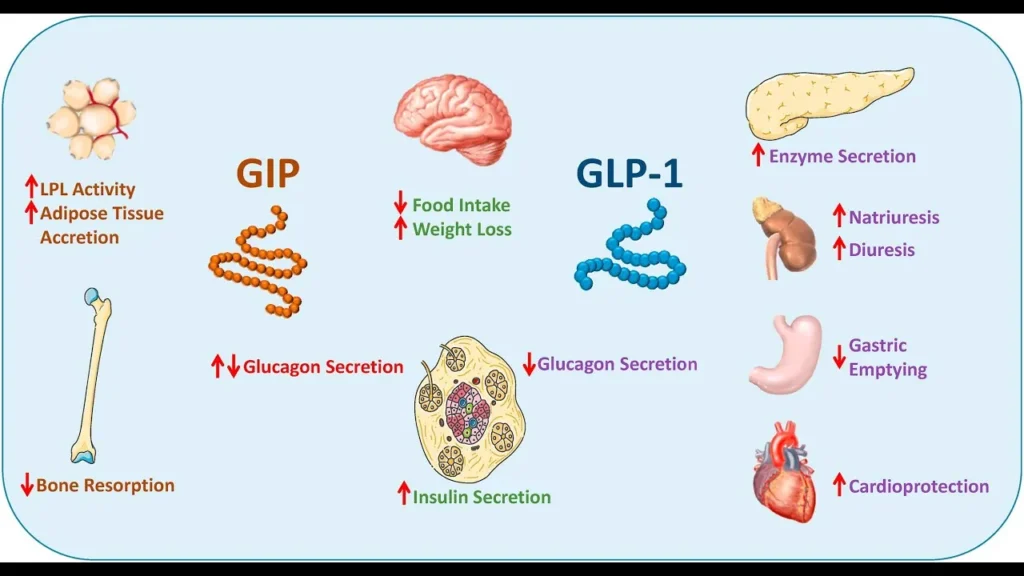
The activation of these receptors leads to:
- Suppression of the centers in the brain that regulate appetite and food cravings.
- Stimulates insulin secretion and reduces glucagon secretion in the pancreas, helping to control blood sugar levels.
- It is also possible that stimulation of GIP receptors causes increased mobilization of fat in adipose tissue, as well as enhanced GLP-1 signaling in the body, thus enhancing its effects.
Thanks to the fatty acid (C20) that is "attached" to the amino acid sequence of Mounjaro, the half-life of the drug is more than five days [3],[4].
Mounjaro Mechanisms of Action
Mounjaro is a powerful tool for simultaneous weight and blood sugar control thanks to its dual GLP-1/GIP action.
According to the available scientific data, this dual receptor activity leads to a synergistic effect that causes more powerful appetite suppression and blood sugar control compared to other medications such as Ozempic or Wegovy, which activate only GLP-1 [5], [6].
Mounjaro has a stronger affinity for GIP receptors than for GLP-1. However, GLP-1 activation is key to the drug's effects and occurs via the cAMP pathway rather than β-arrestin engagement [7]. This means that Munjaro activates GLP-1 without causing habituation.
Clinical trials confirm that, thanks to these mechanisms, it outperforms established therapies for T2D in terms of efficacy and has comparable safety. [8], [9], [10], [11], [12].
Strong activation of GIP receptors may also mediate the significant increase in adiponectin, as observed in some studies—an adipokine associated with the regulation of lipid and glucose metabolism [13, 14].
Increases in serum adiponectin correlate with both weight loss and a lower risk of cardiovascular events [15].
What is Mounjaro FDA-approved for?
Given that Eli Lilly is an American company, the first studies of the drug were conducted here.
After a series of clinical trials, including several phase 3 trials, the US Food and Drug Administration (FDA) approved the drug for T2D and subsequently for a range of other indications.
Specifically, Eli Lilly investigated several applications for tirzepatide and received FDA approvals for various indications in the following chronological order [1, 16, 17, 18, 19]:
- 2016 - Eli Lilly receives a patent for tirzepatide.
- 2022 – FDA approves tirzepatide for the treatment of T2D following successful Phase 3 trials from the SURPASS program; trade name Mounjaro.
- 2023 – The FDA approves tirzepatide for weight loss based on phase 3 trials from the SURMOUNT program; trade name Zepbound. Technically, Zepbound differs from Mounjaro only in its trade name.
- 2024 – The FDA approves Zepbound for the treatment of obstructive sleep apnea (OSA) based on the results of phase 3 clinical trials called SURMOUNT-OSA.
- 2026 – The first detailed results from the phase 3 trial called SURPASS-CVOT are released. The trial investigated the cardiovascular benefits of Mounjaro in diabetics.
On September 15, 2022, shortly after FDA approval, the European Medicines Agency (EMA) also authorized the use of the drug in the European Union.
Specifically, the EMA approved Munjaro for two main indications:
- Treatment of adults with poorly controlled type 2 diabetes mellitus, which can be used alone in patients who cannot take metformin, or in combination with other medications for the treatment of diabetes as an adjunct to diet and exercise.
- For weight control in adults with a BMI ≥ 30 kg/m², or who are overweight (BMI ≥ 27 kg/m² to < 30 kg/m²) with at least one weight-related comorbidity, in combination with a low-calorie diet and increased physical activity.
How effective is Mounjaro for weight loss?
As of 2026, Mounjaro (tirzepatide) is the most powerful weight loss medication approved for use in Europe. This is shown by the results of the aforementioned clinical program SURMOUNT.
It consists of a series of phase 3 studies comparing the drug vs placebo and other drugs such as Ozempic and Wegovy [20].
The main comparison was the percentage of initial weight that patients lost during therapy. In addition, some studies also examined what percentage of weight was regained after treatment discontinuation.
Several studies have been published to date, and you may discover the most impactful ones below:
SURMOUNT-1
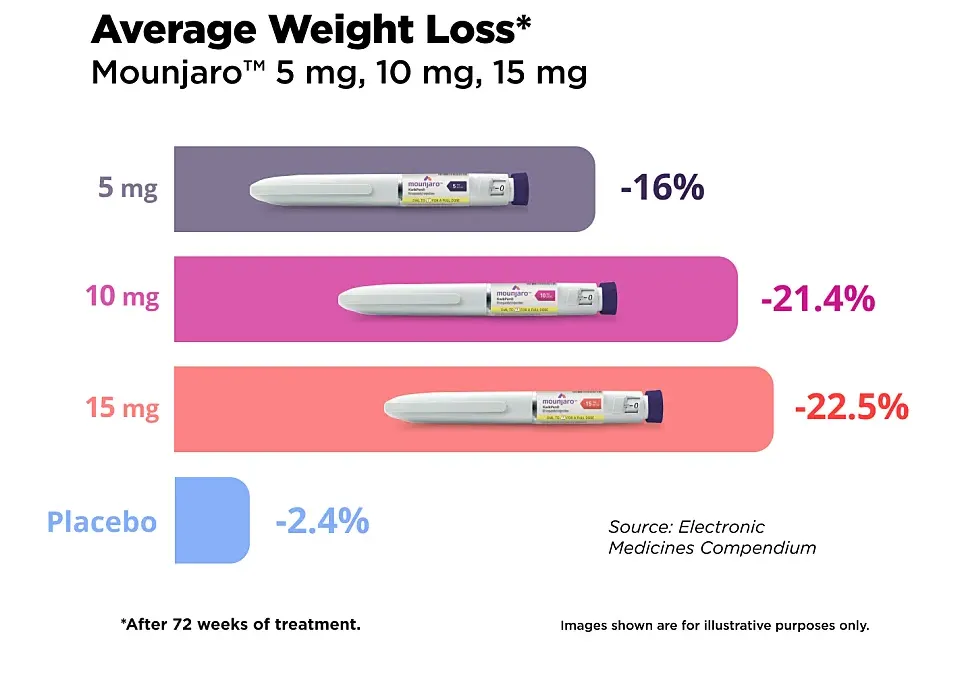
- The results of SURMOUNT-1 were published in The New England Journal of Medicine and present data from over 2,500 participants receiving either a placebo or three different doses of Mundjaro.
- Within 72 weeks, the 5 mg/week group lost an average of 15% of their body weight, while the 10 mg and 15 mg/week groups lost −19.5% and −20.9%, respectively. The results with 10 mg/week are impressive, as the weight loss is almost identical to that with the maximum dose [21].
SURMOUNT-2
- In 2023, the results of SURMOUNT-2 were published in The Lancet. The study assessed the weight loss potential of Mounjaro in 938 people who, in addition to obesity, also had T2D.
- According to the data, tirzepatide at 10 mg/week led to an average weight loss of −12.8% (−12.7 kg), while the 15 mg/week leds to a weight loss of −14.7% (−14.6 kg). The placebo group reported weight loss of −3.3% (−3.2 kg) [22].
SURMOUNT-4
- The longest study in the program is the 88-week SURMOUNT-4 study, which also showed the highest results—an average weight loss of over −25% compared to baseline.
- In fact, patients lose −20.9% (about −22.4 kg) in the first 36 weeks (about 8 months), after which those who continue with Mundjaro lose another −5.5% between weeks 36 and 88.
- Those who stoped the peptide, regained an average of +14% (about +12 kg) between weeks 36 and 88.. It is important to note that 16.6% of patients who stoped taking Munjaro maintained their weight and did not regain any weight during the study period [23].
SURMOUNT-5
- The latest study in the program compared 10 and 15 mg/week of Munjaro with the effectiveness of the highest doses of Wegovy (containing semaglutide, similar to Ozempic), which are 1.7 and 2.4 mg/week.
- Over 72 weeks, the two doses of Munjaro resulted in a mean weight loss of −20.2% from baseline, while the two doses of Vegovi resulted in a mean weight loss of −13.7% from baseline. [24].
Unfortunately, there is no direct comparison between Ozempic vs Mounjarobut it is important to note that Ozempic can contain up to 2mg/weekly semaglutide ..
Due to the even lower dosage of Ozempic vs Wegovy, we can expect Ozempic to also be less powerful for weight loss compared to Mounjaro.
How fast can you lose weight with Mounjaro?
Studies in the SURMOUNT program suggest that weight loss with Mounjaro may be faster at the beginning of the tehrapy and gradually slow down, but it may still continue for up to 88 weeks in some patients.
The most significant weight loss is expected during the first 6-8 months, similar to the weight loss with semaglutide, found in Ozempic and Wegovy [23].
Other metabolic benefits of Mounjaro
In addition to weight loss, Mounjaro has also been approved for the treatment of T2D and obstructive sleep apnea. Moreover, clinical trials are actively investigating Its effectiveness in reducing cardiovascular events.
Effectiveness of Mounjaro in diabetes
Mounjaro was first approved for the treatment of people with type 2 diabetes (T2D). This initial indication reflects how effectively the drug stimulates insulin secretion by activating both GLP-1 and GIP receptors.
Beyond its direct insulinotropic effect, the significant reduction in body fat seen with Mounjaro improves insulin sensitivity. The combination of better insulin action and increased insulin secretion leads to much tighter blood sugar control in people with T2D.
These effects were clearly demonstrated in the first five clinical trials from the SURPASS program, sponsored by the manufacturer Eli Lilly25].
The SURPASS studies compared different doses of tirzepatide (5 mg, 10 mg and 15 mg once weekly) with placebo, Ozempic (semaglutide 1 mg once weekly) and basal insulin.
All tirzepatide doses significantly reduced HbA1c, typically by 2.0–2.5 percentage points below baseline. Even with the 5 mg/week dose, Mounjaro achieved results comparable to Ozempic 1 mg/week and to insulin injections [8], [9], [10], [11], [12].
In two of the studies, tirzepatide 15 mg/week produced an additional HbA1c reduction of 1.66–2.11 percentage points versus placebo [8], [12].
At the 15 mg dose, HbA1c reduction was about 0.5 percentage points greater than with Ozempic (1 mg weekly), 0.9 percentage points greater than with insulin degludec, and 1.0 percentage point greater than with insulin glargine.
How Effective Is Mounjaro for Sleep Apnea?
Mounjaro has generated considerable interest in people who, in addition to obesity, also live with obstructive sleep apnea (OSA).
The key reason is the substantial weight loss achieved with tirzepatide. Patients with OSA tend to lose visceral fat and fat around the pharynx, which reduces the tendency of the upper airway to collapse during sleep.
Weight loss is also associated with lower systemic inflammation (including hsCRP) and reduced blood pressure.
For these reasons, the SURMOUNT clinical program for obesity was expanded with SURMOUNT-OSA [19].
This program includes two parallel, 52-week, randomized, double-blind phase 3 trials - one in patients not using continuous positive airway pressure (CPAP), and one in patients on long-term CPAP therapy.
In both trials, participants were randomized to two arms - Mounjaro, titrated to the maximum tolerated dose (10 mg or 15 mg weekly), or placebo.
The main endpoint was how many times per hour a person temporarily stops breathing during sleep, known as the apnea–hypopnea index (AHI)
With a mean baseline AHI of around 50 events per hour, by week 52 tirzepatide reduced AHI by −25.3 events/hour in the non-CPAP group, and −29.3 events/hour in the CPAP group, whereas placebo reduced AHI by only about −5 to −6 events per hour.
Researchers estimated that this corresponds to a 51–63% reduction in OSA severity. In addition, participants in the tirzepatide groups lost −17.7% and −19.6% of their body weight, compared with only −1.6% and −2.3% in the placebo groups.
There were also statistically significant improvements in hypoxic burden (−61–70%), systolic blood pressure (−3.7 to −7.6 mmHg), and hsCRP.
The most common adverse events were gastrointestinal: diarrhea (26%), nausea (25%), and vomiting (17%), generally mild to moderate in severity.
Discontinuation due to adverse events was under 5% in both study arms.
Based on these data, on December 20, 2024, the FDA approved tirzepatide under the brand name Zepbound as the first medication specifically targeting the underlying cause of moderate-to-severe OSA in people with obesity, after the trials showed an average reduction of about 30 respiratory events per hour.
Is Mounjaro Safe for the Heart?
Current data suggest that Mounjaro is not only safe for the heart, but may also provide meaningful cardiovascular benefits in people with type 2 diabetes [26].
This may be related to GLP-1 signaling, which – together with GIP signaling – promotes weight loss and is thought to have favorable effects on blood pressure and cardiac function. There may also be indirect benefits through reduced inflammation and improved endothelial function.
For example, in a 26-week study in patients with type 2 diabetes, tirzepatide improved cholesterol and triglyceride levels associated with insulin resistance and cardiovascular risk, suggesting a potential preventive effect against cardiovascular disease [27].
Statistical analyses indicate that 15 mg weekly of Mounjaro may reduce the risk of major cardiovascular events by around 20% (hazard ratio [HR] = 0.8) compared with placebo [28].
An ongoing phase 3 trial, SURPASS-CVOT, launched in 2020, is evaluating whether Mounjaro improves long-term cardiovascular outcomes in patients with T2D..
Over 13,000 participants have been enrolled so far and are being followed for several years. Initial results suggest a 28% reduction in the combined risk of heart attack, stroke, and cardiovascular death [17].
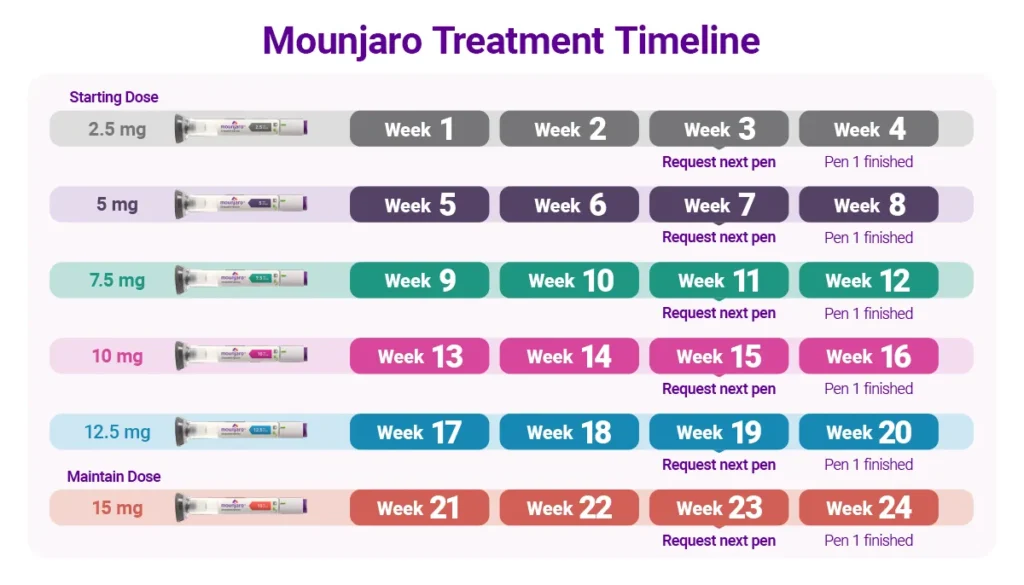
How to dose Moundjaro for weight loss?
Based on the extensive data from the SURPASS and SURMOUNT programs, and the approvals from both the FDA and EMA, we now have clear recommendations for how tirzepatide should be dosed for weight loss [20, 21].
Mounjaro is effective only when given as a subcutaneous injection. In this form, it has a long half-life of about 5–6 days, which allows for once-weekly dosing.
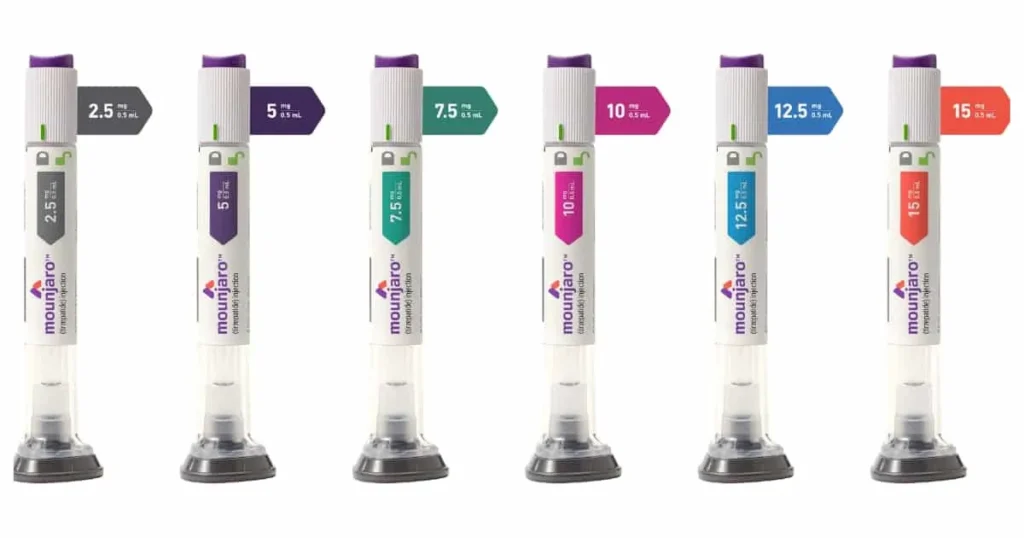
All official sources agree that therapy must start at 2.5 mg once weekly for at least 4 weeks.
After that, the dose is increased in 2.5 mg steps, and each new dose should be maintained for at least 4 weeks before any further increase.
A simple titration schedule looks like this:
- 2.5 mg weekly – at least weeks 1–4
- 5 mg weekly – weeks 5–8
- 7.5 mg weekly – weeks 9–12
- 10 mg weekly – weeks 13–16
- 12.5 mg weekly – weeks 17–20
- 15 mg weekly – from week 21 onward
The maximum dosage does not have to reach 15 mg/weekly and can be maintained at 10 mg/weekly if the patients has already achieved satisfactory results.
It is important to emphasize that there is no need to increase the dose immediately after week 4.
However, it is very important not to increase the dose before at least four injections of the current dose have been given. Doses above 15 mg weekly should not be used, as there are no studies demonstrating safety at higher doses.
Risks and Side Effects of Mounjaro as a Weight-Loss and Diabetes Medication
Mounjaro has been studied in multiple phase 3 clinical trials that examined not only its effectiveness but also its safety profile.
In 2023, researchers pooled and analyzed data from most of these studies to clearly characterize the medication’s safety [29].
Their meta-analysis included about 7,000 participants and showed that side-effect risk is dose-dependent, with most adverse events emerging early in treatment at the lower doses.
Specifically, the reported rates of any side effects at different doses were:
- 5 mg weekly – 39% of participants
- 10 mg weekly – 46%
- 15 mg weekly – 49%
As expected, the highest risk was seen at 15 mg weekly [29]. The most common mild and transient side effects in this group were:
- Nausea – 24%
- Diarrhea – 21%
- Belching – 16%
- Abdominal bloating – 16%
- Abdominal discomfort – 15%
- Vomiting – 14%
- Constipation – 9%
- Indigestion – 9%
- Abdominal pain – 8%
- Elevated pancreatic enzymes – 7%
Serious adverse events were rare, and all affected patients recovered fully:
- Allergic reactions – 3%
- Cholecystitis – under 1%
Is Mounjaro safe?
The most common adverse reactions with Mounjaro involve the gastrointestinal tract and digestion, which is typical for GLP-1 receptor agonists.
The main mechanism is a temporary slowing of gastrointestinal motility, which is most pronounced at the start of treatment and after dose escalations. Because this slowing is transient, the side effects are usually temporary as well.
However, according to the meta-analysis mentioned above, 7–13% of participants discontinued Mounjaro due to side effects [29].
In most cases, these were gastrointestinal issues such as nausea and diarrhea, which can often be prevented or minimized by slow, careful dose escalation.
The overall rate of serious side effects was between 5% and 7%, depending on the dose [29].
The most frequent serious events were related to gallbladder and pancreatic function—problems that can also occur with very rapid weight loss even in the absence of medication.
Regular monitoring of relevant laboratory markers for these organs can help detect emerging issues early.
One important and often underestimated risk is loss of muscle mass, which is a direct consequence of appetite suppression and reduced calorie intake. This can be prevented by combining drug therapy with an appropriate, protein-rich diet and, most importantly, resistance training. most importantly, resistance training..
Concerns about thyroid cancer seen in rodent studies have not been confirmed in humans. Current data suggest that this risk in people is unlikely.
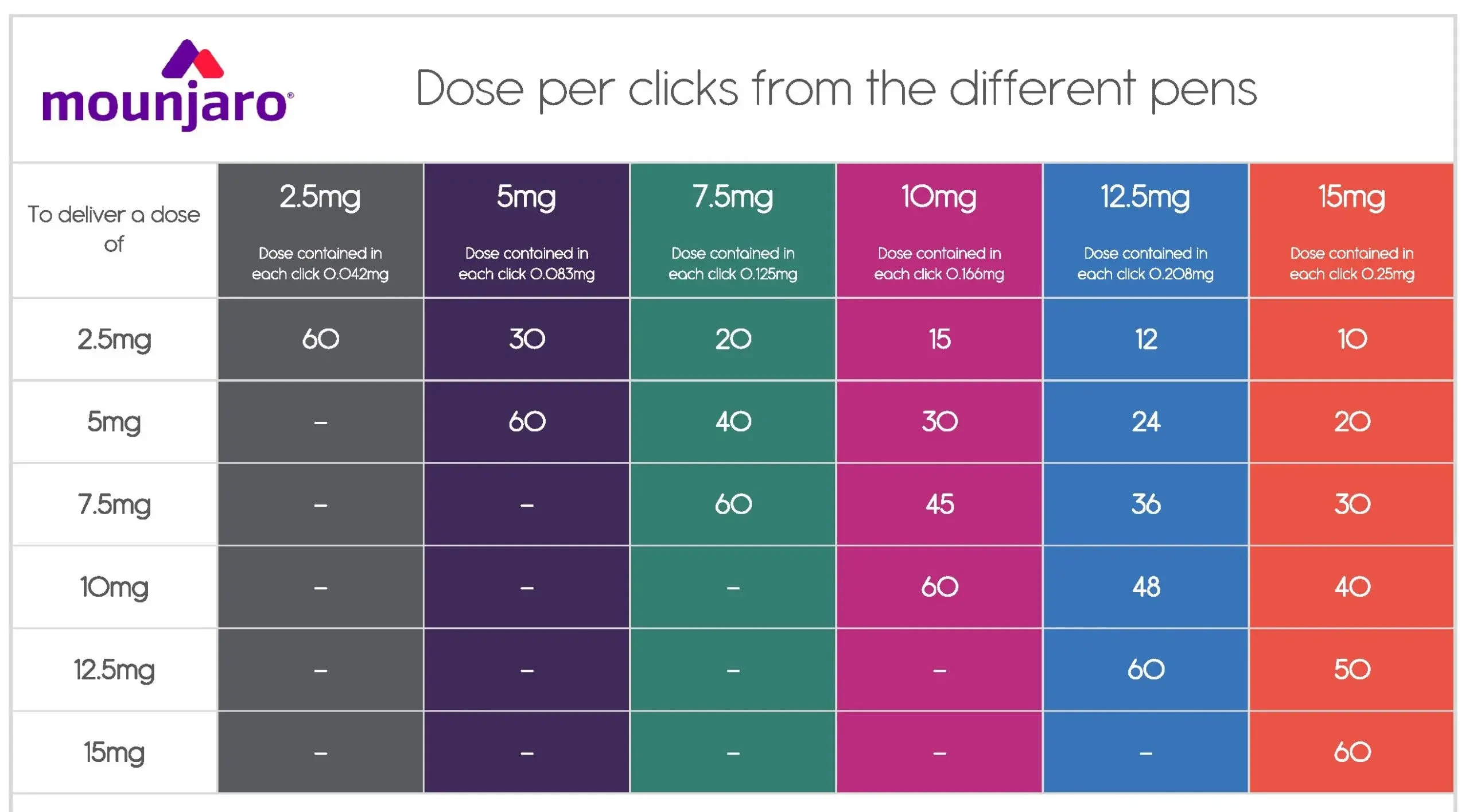
Ако след закупуване на писалка с по-висока седмична доза Мунджаро се появят странични ефекти, които да налагат редуциране на дозата, то няма нужда да закупувате нова, по-малка писалка.
Писалките Мунджаро позволяват „микродозиране“ чрез броене на кликовете, които писалката прави при завъртане на механизма за дозиране.
За това каква точно доза набирате в зависимост от кликовете, може да се ориентирате по следната таблица:
How to store Mounjaro?
Mounjaro (tirzepatide) is a prescription-only injectable medication for subcutaneous use, supplied as a prefilled pen. Each pen contains a ready-to-use tirzepatide solution.
Additional ingredients include sodium phosphate and metacresol in very low concentrations, which stabilize the solution and inhibit microbial growth.
Thanks to this formulation, a pen remains usable for more than four weeks after first use.
Ideally, Mounjaro should be stored in a refrigerator at 36°F to 46°F (2–8°C) until the expiration date indicated on the package.
After the first use, the pen may be kept at room temperature (up to 86°F / 30°C) for up to 28 days. Continued refrigerated storage after opening may help preserve stability for this period.
Do not freeze, shake, or drop the pen, and do not expose it to direct sunlight or heat, as this can degrade the peptide and reduce the amount and effectiveness of active tirzepatide.
How is Mounjaro injected?
Mounjaro must be administered only as a subcutaneous injection. The drug is not effective if injected by other routes or taken orally.
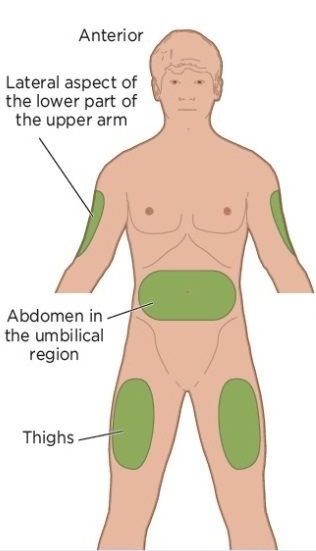
Preferred Injection Sites
The most convenient sites for patients are the abdomen (at least 2 inches / 5 cm to the left or right of the navel), the upper outer area of the arm, or the outer part of the thigh.
The abdominal area is usually preferred because the skin is thicker there and injections tend to be less uncomfortable.
The injection site should be rotated by at least 0.5–1 inch (1–2 cm) each week to avoid local tissue irritation.
Avoid skin that is damaged, red, irritated, or infected.
How to inject Mounjaro?
What You Need:
- Cotton balls or cotton pads
- 70% ethyl alcohol
Step-by-Step Injection Technique
- Remove the pen from the refrigerator and visually inspect the solution. It should be clear, without particles or sediment.
- Clean the chosen injection site with an alcohol-soaked cotton pad and let it dry.
- Set the dose on the pen, remove the protective cap, and attach the needle.
- The needle has two small caps – remove both.
- Insert the needle into the skin at a 90-degree angle and press the injection button. Hold the button for about 10 seconds to ensure the full dose is delivered.
- Remove the pen and gently press the site with an alcohol-soaked cotton pad without massaging.
- Replace the large cap on the needle, unscrew it, and dispose of it safely.
- Put the protective cap back on the pen.
Mild, temporary reactions like redness, swelling, or slight discomfort at the injection site are possible and typically resolve quickly.
If a small lump appears, it usually means the injection was given too superficially. It should disappear within 24 hours and is not known to affect the drug’s effectiveness.
How Long Can Mounjaro Be Used?
Clinical studies with tirzepatide show that it can be used safely over a prolonged period (more than 88 weeks). Excellent blood sugar control and weight-loss effects are usually seen within the first several months of therapy, around 32 weeks.
How Much Does Mounjaro Cost?
In the European pharmacy network, the currently available doses are 2.5 mg, 5 mg, 7.5 mg, and 10 mg weekly. Doses of 12.5 mg and 15 mg weekly are not yet available. 7.5 мг/седмица и 10 мг/седмица. Дози от 12.5 мг Мунджаро на седмица и 15 мг/седмица засега липсват в България.
As of 2026, price checks in several pharmacies show approximate costs of 200+ EUR for 2.5 mg weekly, 250+ EUR for 5 mg weekly, and 350+ EUR for 7.5 mg and 10 mg weekly.
For comparison, this is roughly 2–3 times higher than the price of Ozempic (0.25, 0.5, or 1 mg weekly), and slightly higher than that of Wegovy.
Mounjaro – A Physician's Opinion on Safety and Effectiveness
Mounjaro is both effective and safe when prescribed according to medical indications and used under regular specialist supervision.
At present, Mounjaro is the most effective weight-loss medication on the European market, while having a safety profile similar to that of Ozempic.
The most common side effects are gastrointestinal, such as nausea and diarrhea, which tend to be most intense at the beginning of therapy and improve over time.
Patients with a history or high risk of pancreatitis or gallbladder disease require extra caution and regular monitoring.
In people with diabetes, transient visual disturbances may occur due to rapid improvement in blood sugar control. This typically resolves within a few weeks.
So far, there is no clinical evidence that tirzepatide causes thyroid disease, cancer, or kidney damage. These events occur with a frequency similar to that in placebo groups.
It is crucial that Mounjaro be combined with an appropriate diet and physical activity. This not only improves treatment effectiveness but also helps prevent loss of muscle mass, which is a potentially serious side effect with long-term therapy.
— Dimitar Marinov, MD, PhD
If you found this article helpful, feel free to follow me on social media for more evidence-based content.
References:
- Chavda VP, Ajabiya J, Teli D, Bojarska J, Apostolopoulos V. Tirzepatide, a New Era of Dual-Targeted Treatment for Diabetes and Obesity: A Mini-Review. Molecules. 2022 Jul 5;27(13):4315. doi: 10.3390/molecules27134315. Erratum in: Molecules. 2025 Mar 07;30(6):1190. doi: 10.3390/molecules30061190. PMID: 35807558; PMCID: PMC9268041.
- Seino Y, Fukushima M, Yabe D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J Diabetes Investig. 2010 Apr 22;1(1-2):8-23. doi: 10.1111/j.2040-1124.2010.00022.x. PMID: 24843404; PMCID: PMC4020673.
- Sun B, Willard FS, Feng D, Alsina-Fernandez J, Chen Q, Vieth M, Ho JD, Showalter AD, Stutsman C, Ding L, Suter TM, Dunbar JD, Carpenter JW, Mohammed FA, Aihara E, Brown RA, Bueno AB, Emmerson PJ, Moyers JS, Kobilka TS, Coghlan MP, Kobilka BK, Sloop KW. Structural determinants of dual incretin receptor agonism by tirzepatide. Proc Natl Acad Sci U S A. 2022 Mar 29;119(13):e2116506119. doi: 10.1073/pnas.2116506119. Epub 2022 Mar 25. PMID: 35333651; PMCID: PMC9060465.
- Zhao F, Zhou Q, Cong Z, Hang K, Zou X, Zhang C, Chen Y, Dai A, Liang A, Ming Q, Wang M, Chen LN, Xu P, Chang R, Feng W, Xia T, Zhang Y, Wu B, Yang D, Zhao L, Xu HE, Wang MW. Structural insights into multiplexed pharmacological actions of tirzepatide and peptide 20 at the GIP, GLP-1 or glucagon receptors. Nat Commun. 2022 Feb 25;13(1):1057. doi: 10.1038/s41467-022-28683-0. PMID: 35217653; PMCID: PMC8881610.
- Coskun T, Sloop KW, Loghin C, Alsina-Fernandez J, Urva S, Bokvist KB, Cui X, Briere DA, Cabrera O, Roell WC, Kuchibhotla U, Moyers JS, Benson CT, Gimeno RE, D’Alessio DA, Haupt A. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol Metab. 2018 Dec;18:3-14. doi: 10.1016/j.molmet.2018.09.009. Epub 2018 Oct 3. PMID: 30473097; PMCID: PMC6308032.
- Min T, Bain SC. The Role of Tirzepatide, Dual GIP and GLP-1 Receptor Agonist, in the Management of Type 2 Diabetes: The SURPASS Clinical Trials. Diabetes Ther. 2021 Jan;12(1):143-157. doi: 10.1007/s13300-020-00981-0. Epub 2020 Dec 15. PMID: 33325008; PMCID: PMC7843845.
- Willard FS, Douros JD, Gabe MB, Showalter AD, Wainscott DB, Suter TM, Capozzi ME, van der Velden WJ, Stutsman C, Cardona GR, Urva S, Emmerson PJ, Holst JJ, D’Alessio DA, Coghlan MP, Rosenkilde MM, Campbell JE, Sloop KW. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight. 2020 Sep 3;5(17):e140532. doi: 10.1172/jci.insight.140532. PMID: 32730231; PMCID: PMC7526454.
- Rosenstock J, Wysham C, Frías JP, Kaneko S, Lee CJ, Fernández Landó L, Mao H, Cui X, Karanikas CA, Thieu VT. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021 Jul 10;398(10295):143-155. doi: 10.1016/S0140-6736(21)01324-6. Epub 2021 Jun 27. Erratum in: Lancet. 2021 Jul 17;398(10296):212. doi: 10.1016/S0140-6736(21)01556-7. PMID: 34186022.
- Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, Liu B, Cui X, Brown K; SURPASS-2 Investigators. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N Engl J Med. 2021 Aug 5;385(6):503-515. doi: 10.1056/NEJMoa2107519. Epub 2021 Jun 25. PMID: 34170647.
- Ludvik B, Giorgino F, Jódar E, Frias JP, Fernández Landó L, Brown K, Bray R, Rodríguez Á. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021 Aug 14;398(10300):583-598. doi: 10.1016/S0140-6736(21)01443-4. Epub 2021 Aug 6. PMID: 34370970.
- Del Prato S, Kahn SE, Pavo I, Weerakkody GJ, Yang Z, Doupis J, Aizenberg D, Wynne AG, Riesmeyer JS, Heine RJ, Wiese RJ; SURPASS-4 Investigators. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021 Nov 13;398(10313):1811-1824. doi: 10.1016/S0140-6736(21)02188-7. Epub 2021 Oct 18. PMID: 34672967.
- Dahl D, Onishi Y, Norwood P, Huh R, Bray R, Patel H, Rodríguez Á. Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients With Type 2 Diabetes: The SURPASS-5 Randomized Clinical Trial. JAMA. 2022 Feb 8;327(6):534-545. doi: 10.1001/jama.2022.0078. PMID: 35133415; PMCID: PMC8826179.
- Ali R, Virendra SA, Chawla PA. Bumps and humps in the success of Tirzepatide as the first GLP1 and GIP receptor agonist. Health Sciences Review. 2022 Sep 1;4:100032.
- Thomas MK, Nikooienejad A, Bray R, Cui X, Wilson J, Duffin K, Milicevic Z, Haupt A, Robins DA. Dual GIP and GLP-1 Receptor Agonist Tirzepatide Improves Beta-cell Function and Insulin Sensitivity in Type 2 Diabetes. J Clin Endocrinol Metab. 2021 Jan 23;106(2):388-396. doi: 10.1210/clinem/dgaa863. PMID: 33236115; PMCID: PMC7823251.
- Yanai H, Yoshida H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. Int J Mol Sci. 2019 Mar 8;20(5):1190. doi: 10.3390/ijms20051190. PMID: 30857216; PMCID: PMC6429491.
- Tirzepatide (Zepbound) for chronic weight management. Med Lett Drugs Ther. 2023 Dec 25;65(1692):205-207. doi: 10.58347/tml.2023.1692c. PMID: 38133594.
- Fadini G. P. (2025). Can Dual Incretin Receptor Agonists Exert Better Cardiovascular Protection than Selective GLP-1 Receptor Agonists? Highlights from SURPASS-CVOT. Diabetes therapy : research, treatment and education of diabetes and related disorders, 16(10), 1893–1898. https://doi.org/10.1007/s13300-025-01784-x
- Anderer S. FDA Approves Tirzepatide as First Drug for Obstructive Sleep Apnea. JAMA. 2025 Feb 25;333(8):656. doi: 10.1001/jama.2024.28055. PMID: 39853991.
- Malhotra A, Grunstein RR, Fietze I, Weaver TE, Redline S, Azarbarzin A, Sands SA, Schwab RJ, Dunn JP, Chakladar S, Bunck MC, Bednarik J; SURMOUNT-OSA Investigators. Tirzepatide for the Treatment of Obstructive Sleep Apnea and Obesity. N Engl J Med. 2024 Oct 3;391(13):1193-1205. doi: 10.1056/NEJMoa2404881. Epub 2024 Jun 21. Erratum in: N Engl J Med. 2024 Oct 17;391(15):1464. doi: 10.1056/NEJMx240005. PMID: 38912654; PMCID: PMC11598664.
- le Roux CW, Zhang S, Aronne LJ, Kushner RF, Chao AM, Machineni S, Dunn J, Chigutsa FB, Ahmad NN, Bunck MC. Tirzepatide for the treatment of obesity: Rationale and design of the SURMOUNT clinical development program. Obesity (Silver Spring). 2023 Jan;31(1):96-110. doi: 10.1002/oby.23612. Epub 2022 Dec 7. PMID: 36478180; PMCID: PMC10107501.
- Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, Kiyosue A, Zhang S, Liu B, Bunck MC, Stefanski A; SURMOUNT-1 Investigators. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med. 2022 Jul 21;387(3):205-216. doi: 10.1056/NEJMoa2206038. Epub 2022 Jun 4. PMID: 35658024.
- Garvey WT, Frias JP, Jastreboff AM, le Roux CW, Sattar N, Aizenberg D, Mao H, Zhang S, Ahmad NN, Bunck MC, Benabbad I, Zhang XM; SURMOUNT-2 investigators. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2023 Aug 19;402(10402):613-626. doi: 10.1016/S0140-6736(23)01200-X. Epub 2023 Jun 26. PMID: 37385275.
- Aronne LJ, Sattar N, Horn DB, Bays HE, Wharton S, Lin WY, Ahmad NN, Zhang S, Liao R, Bunck MC, Jouravskaya I, Murphy MA; SURMOUNT-4 Investigators. Continued Treatment With Tirzepatide for Maintenance of Weight Reduction in Adults With Obesity: The SURMOUNT-4 Randomized Clinical Trial. JAMA. 2024 Jan 2;331(1):38-48. doi: 10.1001/jama.2023.24945. PMID: 38078870; PMCID: PMC10714284.
- Aronne LJ, Horn DB, le Roux CW, Ho W, Falcon BL, Gomez Valderas E, Das S, Lee CJ, Glass LC, Senyucel C, Dunn JP; SURMOUNT-5 Trial Investigators. Tirzepatide as Compared with Semaglutide for the Treatment of Obesity. N Engl J Med. 2025 May 11. doi: 10.1056/NEJMoa2416394. Epub ahead of print. PMID: 40353578.
- https://investor.lilly.com/news-releases/news-release-details/fda-approves-lillys-mounjarotm-tirzepatide-injection-first-and
- Tate M, Chong A, Robinson E, Green BD, Grieve DJ. Selective targeting of glucagon-like peptide-1 signalling as a novel therapeutic approach for cardiovascular disease in diabetes. Br J Pharmacol. 2015 Feb;172(3):721-36. doi: 10.1111/bph.12943. Epub 2014 Dec 1. PMID: 25231355; PMCID: PMC4301685.
- Wilson JM, Nikooienejad A, Robins DA, Roell WC, Riesmeyer JS, Haupt A, Duffin KL, Taskinen MR, Ruotolo G. The dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist, tirzepatide, improves lipoprotein biomarkers associated with insulin resistance and cardiovascular risk in patients with type 2 diabetes. Diabetes Obes Metab. 2020 Dec;22(12):2451-2459. doi: 10.1111/dom.14174. Epub 2020 Sep 15. PMID: 33462955; PMCID: PMC7756479.
- Sattar N, McGuire DK, Pavo I, Weerakkody GJ, Nishiyama H, Wiese RJ, Zoungas S. Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Nat Med. 2022 Mar;28(3):591-598. doi: 10.1038/s41591-022-01707-4. Epub 2022 Feb 24. PMID: 35210595; PMCID: PMC8938269.
- Mishra R, Raj R, Elshimy G, Zapata I, Kannan L, Majety P, Edem D, Correa R. Adverse Events Related to Tirzepatide. J Endocr Soc. 2023 Jan 26;7(4):bvad016. doi: 10.1210/jendso/bvad016. PMID: 36789109; PMCID: PMC9915969.

6 thoughts on “Мунджаро за Отслабване и Лечение на Диабет Тип 2 – Мнение на Лекар”
Вие изписвате ли го?
Здравейте, да, изписвам го.
Аз имам диабет тип 2 и пълнея постоянно , това лекарство , дали ще ми помогне да сваля килограми.
Да, най-вероятно ще помогне.
Здравейте, след като съм на Оземпик 2 мг и не отслабвам и гликираният ми хемоглобин не спада, на каква доза Мунджаро трябва да премина. Личния лекар казва направо на 10 мг, но тук пише че въпреки високата ми доза Оземпик трябва да започна с 2,5 мг.
Аз предлагам да започнете с 5мг или 7.5мг по-скоро, но въпроса е съвсем друг – каква е диетата и какви са й проблемите, че не се повлияват от медикамента.
Няма как да очаквате ефект, от който и да от медикаментите, ако не е обърнато внимание индивидуално към диетата (вкл. заетост, предпочитания), и не говоря за общи препоръки като тези от листовките за ДТ2 тип „не яжте захар“. Нужен е индивидуализиран подход.